Importation des protéines et biogenèse des chloroplastes
TIC et TOC - ou comment les protéines entrent dans les chloroplastes
| Professeur | Prof. Felix Kessler |
| Post-Doc | Dr Shanmugabalaji Venkatasalam |
| Doctorants |
Gent Ballabani ; Maryam Forough |
| Master | Mondragon Bruno |
Light triggers a developmental program in plants that leads to photoautotrophic capacity. A key step in this process is the biogenesis of chloroplasts from undifferentiated proplastids. The assembly of the photosynthetic apparatus requires the import of approximately 2,000 different nuclear encoded proteins. Nuclear encoded proteins are synthesized in the cytosol as precursors with an N-terminal targeting peptide (transit peptide) and must be imported into the nascent chloroplast.
 |
Development of etioplasts (leff) to chloroplasts (right). Picture : T. Kleffmann (Institute of Plant Science ETH Zürich) (Click to enlarge) |
The chloroplast is enclosed by an envelope consisting of two membranes. Both membranes contain translocons to facilitate the import of precursor proteins. These are termed the Toc- (translocon at the outer chloroplast membrane) and Tic-complexes (translocon at the inner chloroplast membrane). The Toc-complex consists of three major components forming a stable complex. Toc159 (the number indicates the molecular mass in kD) and Toc34are surface exposed, GTP-binding integral membrane proteins. The two proteins share a highly conserved GTP-binding domain. The available evidence indicates that the two proteins act in concert to recognize the chloroplast targeting peptide. Toc159 is thought to act as the primary receptor of transit sequences. An alternative model in which Toc34 functions as the primary receptor and Toc159 as a GTP-dependent translocation motor has also been proposed. Toc75, the third component, forms at least part of a hydrophilic channel through which precursors are translocated across the outer membrane. The work in our lab is aimed at the elucidation of Toc-GTPase function using a combination of in vivo and in vitro methods.
| Model for protein import in chloroplasts. In blue, the cytosol. In white, the chloroplast envelope. In green, the stoma. (click to enlarge) |
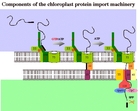 |
The evidences accumulated by a number of groups suggest the following model for the chloroplast protein import machinery: the precursor protein is synthesized in the cytosol with an N-terminal transit peptide. In a first energy independent step the precursor binds to Toc159.In a second step requiring ATP and GTP, the precursor traverses the outer membrane and meets the components of the Tic-complex at the inner membrane surface. In a third step requiring ATP, the precursor translocates across both membrane simultaneously.
What is the physiological significance of the components of the chloroplast
protein import machinery ?
We recently isolated an Arabidopsis mutant, termed ppi2(plastid protein import mutant 2), which lacks atToc159. Compared to wild type chloroplasts, ppi2 plastids resemble undifferentiated proplastids lacking thylakoid membranes and starch granules.
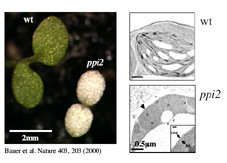 |
Phenotype of the Arabidopsis ppi2 mutant. (click to enlarge) |
We analyzed the distribution of atToc159 between the soluble and chloroplast fraction. To our surprise, atToc159 was present in both fractions. Transient expression of a GFP-fusion in Arabidopsis protoplasts supports this finding. We can see that the GFP-fusion is concentrated at the chloroplast surface, but is also present in the cytosol surrounding the vacuole.
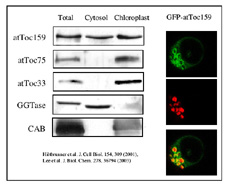 |
Soluble and outer membrane forms of Toc159 The data shows that atToc159 exists both in a soluble and membrane bound form. Toc159 may serve not only as a membrane receptor but also as a soluble receptor.We hypothesize that atToc159 may cycle between the two forms and that this cycle may be controlled by GTP. (click to enlarge) |
Model for the initiation of precursor import at the chloroplast outer membrane. 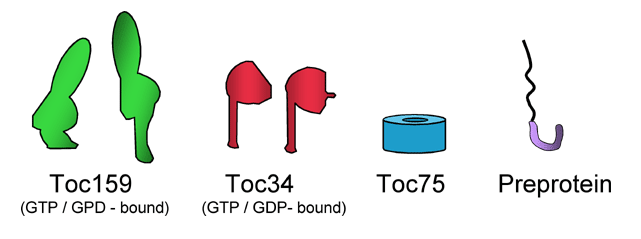
|
Tic110, -62, -55, -40, -22 and -20 have been identified as components of the import machinery at the inner envelope membrane. Tic110was found in association with a late translocation intermediate, spanning both envelope membranes, suggesting a role in the terminal stages of import. Tic55 and Tic62 co-isolated with Tic110 in a complex. Both proteins contain redox elements suggesting that protein import may be controlled by the redox potential of chloroplasts. Finally, Tic20 and Tic22crosslinked to precursor proteins stably inserted across the outer chloroplast membrane, but not yet having reached the chloroplast stroma. The data suggest that Tic20 and Tic22 initiate the translocation of the incoming precursor across the inner membrane. In vivo analysis of the Tic20 ortholog in Arabidopsis, atTic20, demonstrates a role for this protein in the translocation of preproteins across the inner envelope membrane.
Though Tic110 is a widely accepted component of the import machinery, controversy surrounds the topology and function of Tic110. Tic110 is integral membrane protein anchored by a transmembrane domain consisting of two predicted transmembrane helices near the N-terminus of the protein. The large approx. 90 kD C-terminus is predicted to be largely a-helical and not to contain membrane-anchoring hydrophobic a-helices. Conflicting reports have been published regarding the topology of Tic110, reaching the conclusion that the C-terminal domain extends either into the stroma or the intermembrane space between the two envelope membranes. The reported interactions with the stromal chaperones, cpn60 and ClpC, suggest that Tic110 may function to couple the import of proteins to their folding. Moreover, ClpC has been proposed to function as a motor driving protein transport across the inner membrane. An alternative function for Tic110 has also been put forward. Recombinant Tic110, incorporated into lipid bilayers showed channel activity influenced by precursor proteins. The channel function is attributed to the C-terminal domain and infers that it contains transmembrane segments. Our current research focuses on the in vivo and biochemical function of atTic110.


